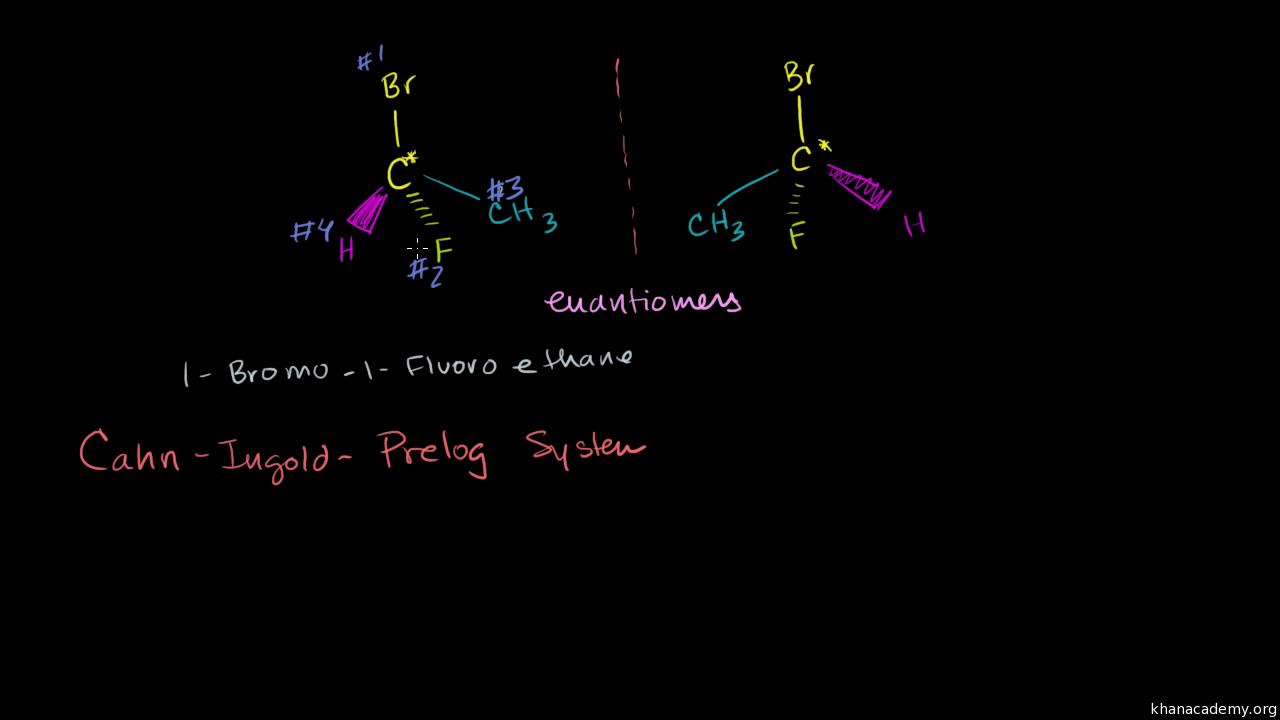
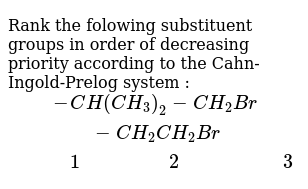
Cahn-ingold-prelog System
Cahn-ingold-prelog system. A strong base is not required to form the alkene since there is no leaving group that will need to be displaced more on that in a second. Conversely a mirror image of an achiral object such as a sphere. The ambiguity comes from the definition of similar groups.
The word chirality is derived from the Greek χειρ kheir hand a familiar chiral object. Vor allem bei Kohlenstoffverbindungen ist es oft schwierig die räumliche Ausrichtung der bis zu vier Bindungspartner im Tetraederwinkel von 1095 deutlich zu machen. The cis-trans definition is unambiguous only when you have two different groups on one of the alkene carbons and the same two groups on the other carbon as in but-2-ene.
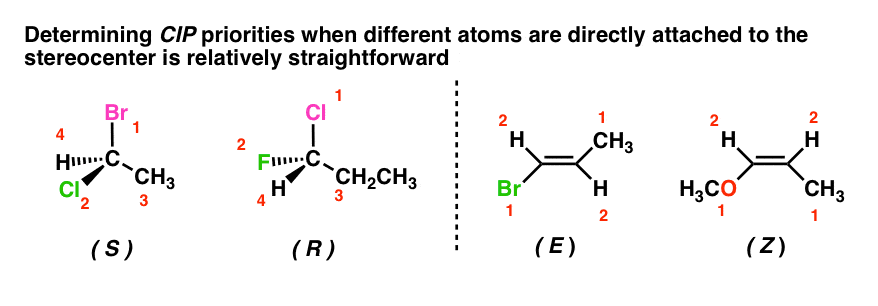
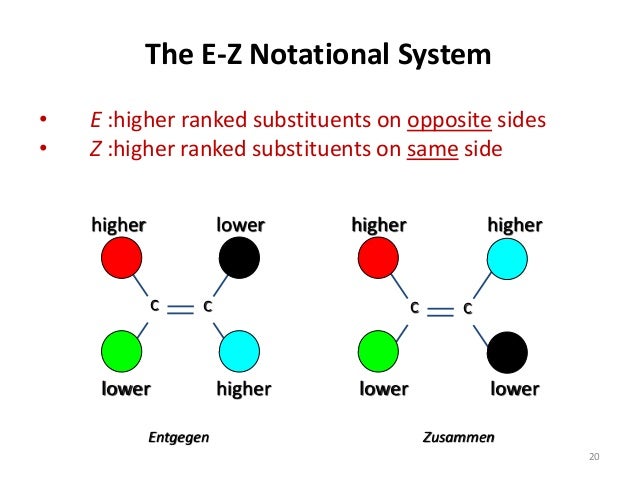
In that case cis and trans- is redundant. The general strategy of the E-Z system is to analyze the two groups at each end of the double bond. The rigorous IUPAC system for naming alkene isomers called the E-Z system is based on the same priority rulesThese priority rules are often called the Cahn-Ingold-Prelog CIP rules after the chemists who developed the system.
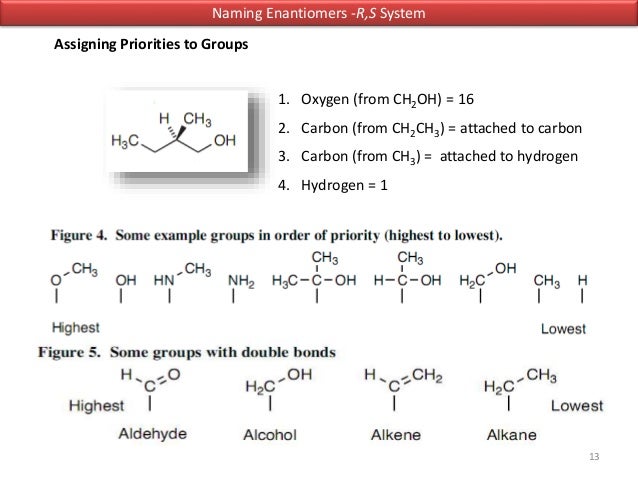
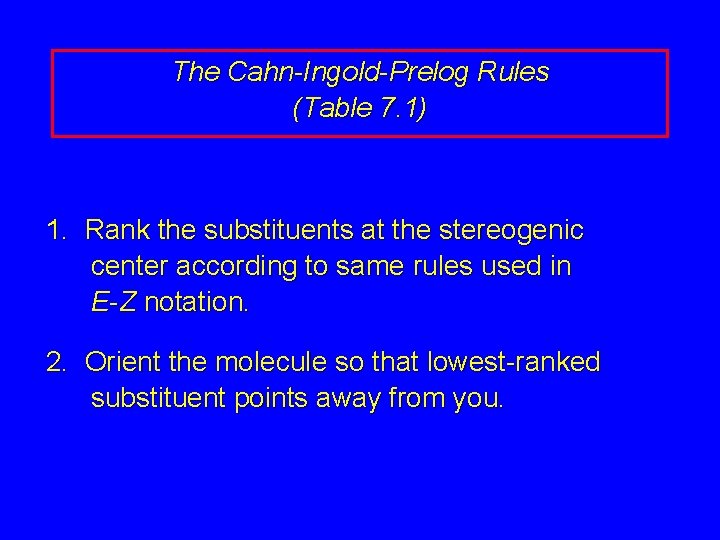
Then the two identical methyl groups are either cis or trans to each other and the two identical hydrogen atoms are either cis or trans to each other. This system known as the Cahn-Ingold-Prelog rules uses and elaborates the priority rules developed earlier. An object or a system is chiral if it is distinguishable from its mirror image.
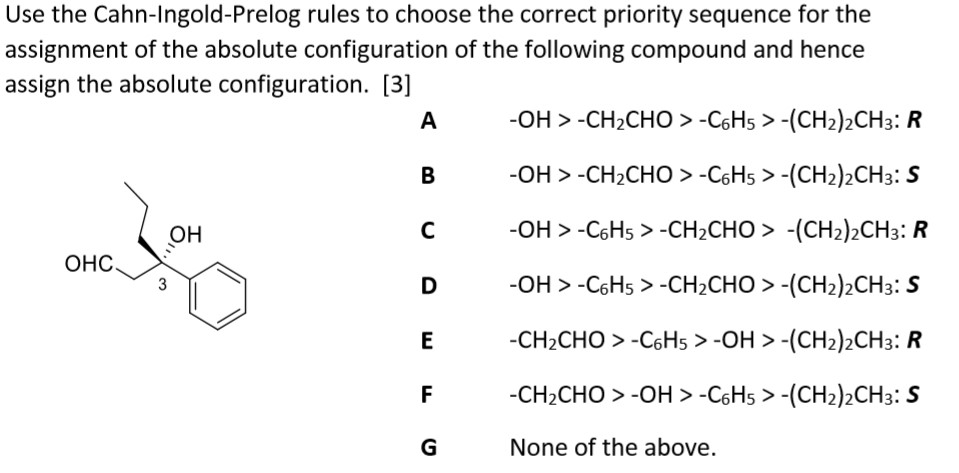
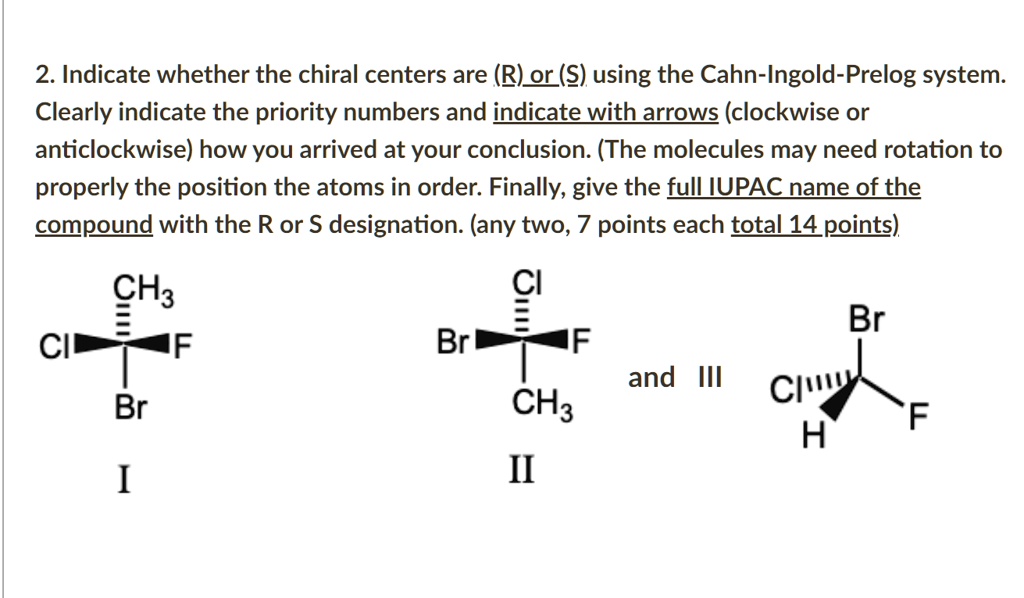
Revisaremos qué hace a una molécula quiral los estereoisómetros la asignación de configuraciones usando el sistema R S la actividad óptica y las proyecciones de Fischer. 26 The purpose of the CIP system is to assign an R or S. Bei vielen chemischen Verbindungen ist die Stellung der Atome im Raum entscheidend für ihre Eigenschaften was eine Unterscheidung verschiedener Stereoisomere notwendig macht.
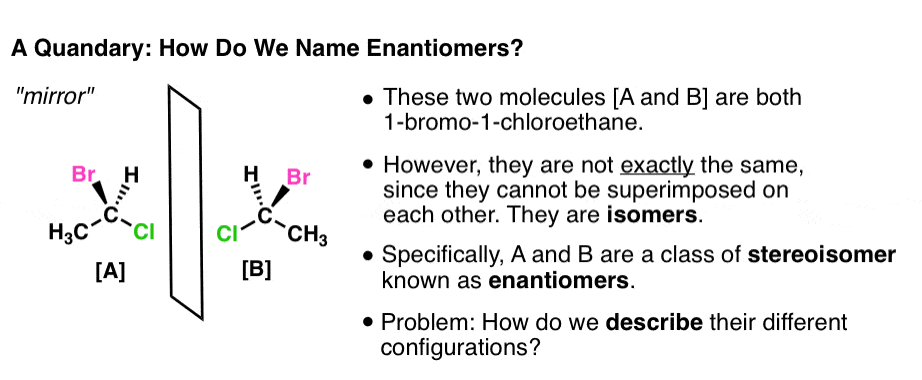
Esta propiedad se llama quiralidad. An alternative system which specifies the absolute configuration of substituted carbon atoms may also be used. The rate of the E1 reaction depends only on the substrate since the rate limiting step is the formation of a carbocationHence the more stable that carbocation is the faster the reaction will be.
Forming the carbocation is the slow step. That is it cannot be superimposed onto it.
In that case cis and trans- is redundant.
In that case cis and trans- is redundant. The ambiguity comes from the definition of similar groups. Die Fischer-Projektion löst dieses Problem ohne. Así como a tu pie izquierdo no le queda bien tu zapato derecho las moléculas también tienen propiedades que dependen de su lateralidad. The rate of the E1 reaction depends only on the substrate since the rate limiting step is the formation of a carbocationHence the more stable that carbocation is the faster the reaction will be. An object or a system is chiral if it is distinguishable from its mirror image. That is it cannot be superimposed onto it. Esta propiedad se llama quiralidad. This page is the property of William Reusch.
If we want to specify a particular enantiomer we need to use the Cahn-Ingold-Prelog CIP system of assigning R and S configurations which provides us with the absolute configuration. A strong base is not required to form the alkene since there is no leaving group that will need to be displaced more on that in a second. This page is the property of William Reusch. Die Fischer-Projektion löst dieses Problem ohne. That is it cannot be superimposed onto it. Revisaremos qué hace a una molécula quiral los estereoisómetros la asignación de configuraciones usando el sistema R S la actividad óptica y las proyecciones de Fischer. The general strategy of the E-Z system is to analyze the two groups at each end of the double bond.
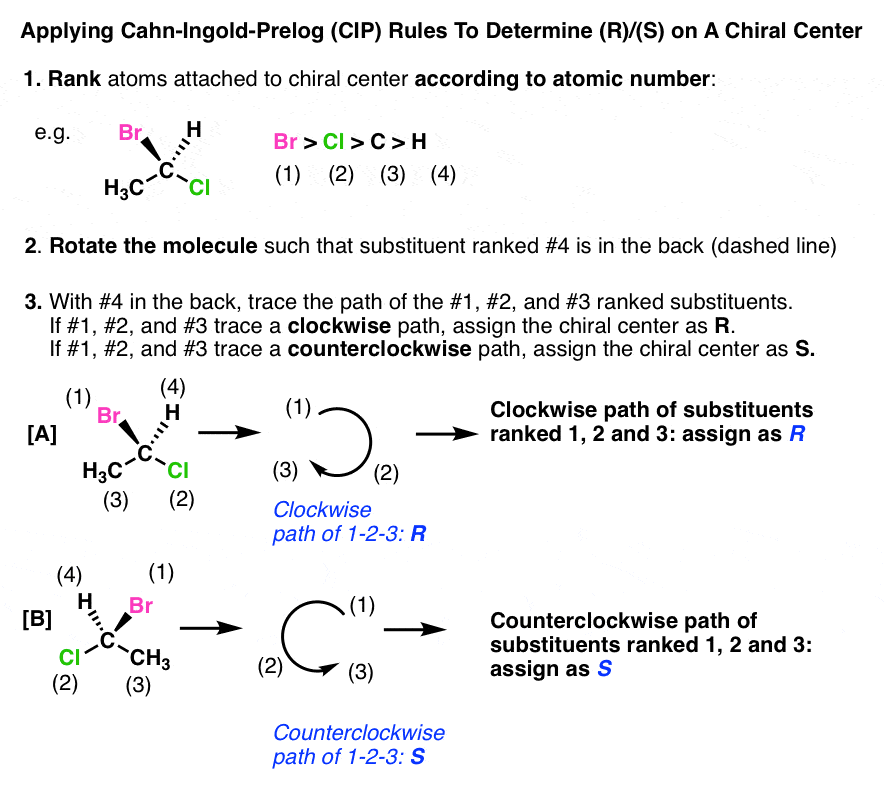
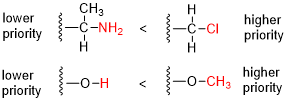
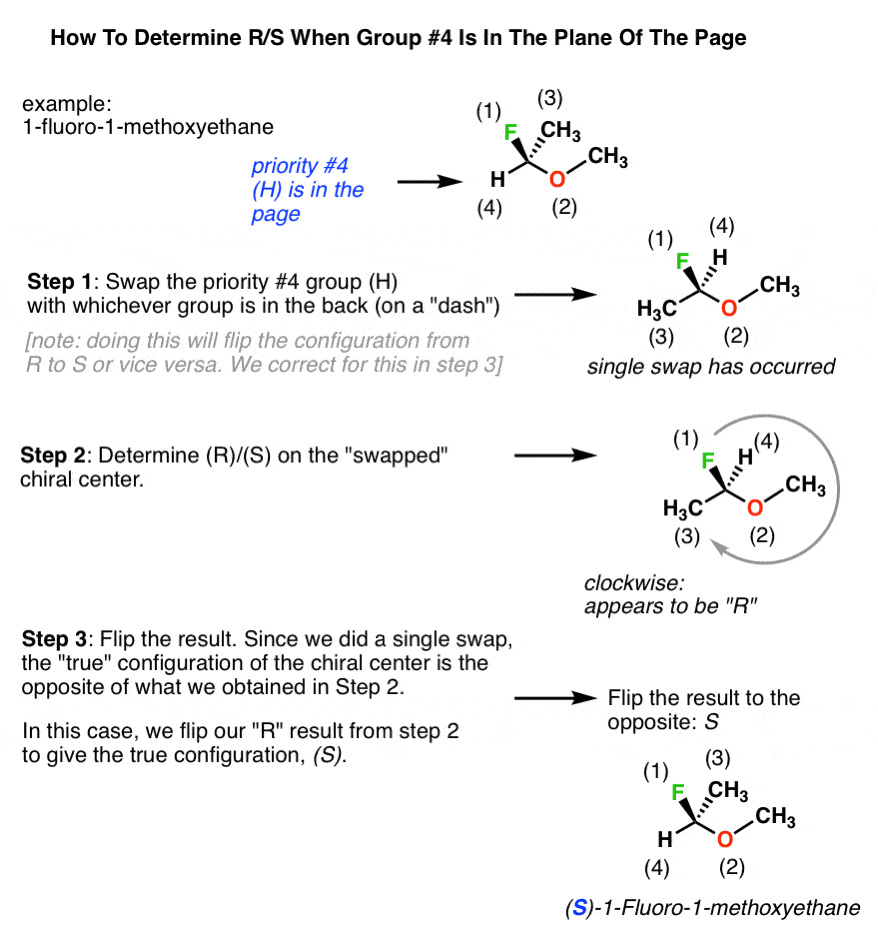



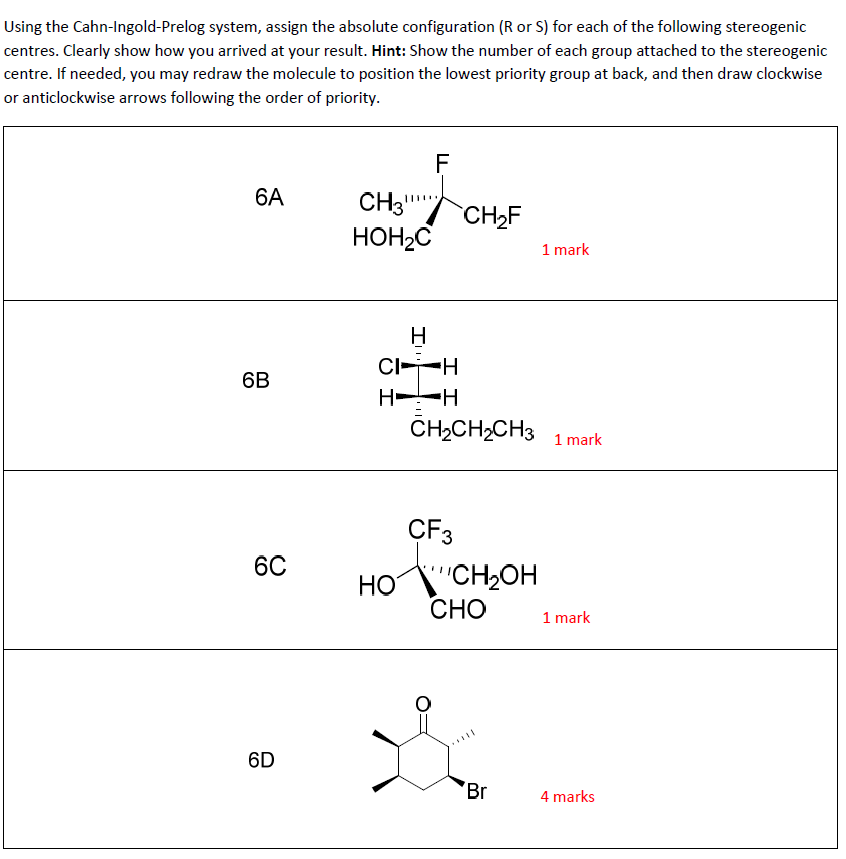











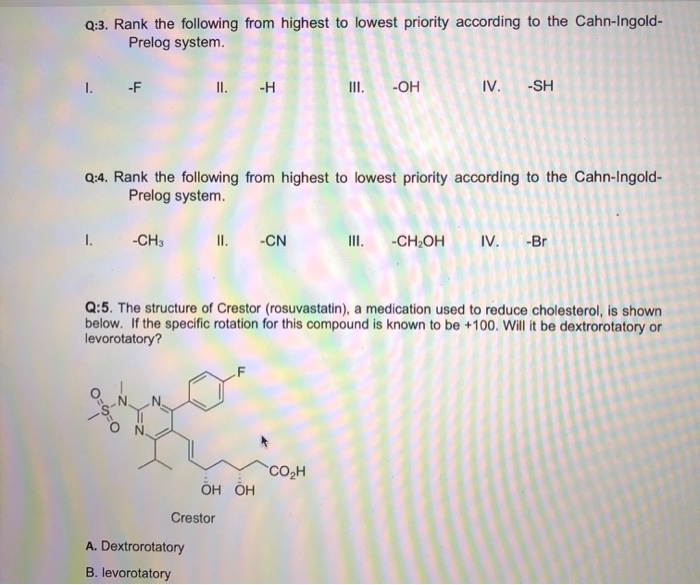
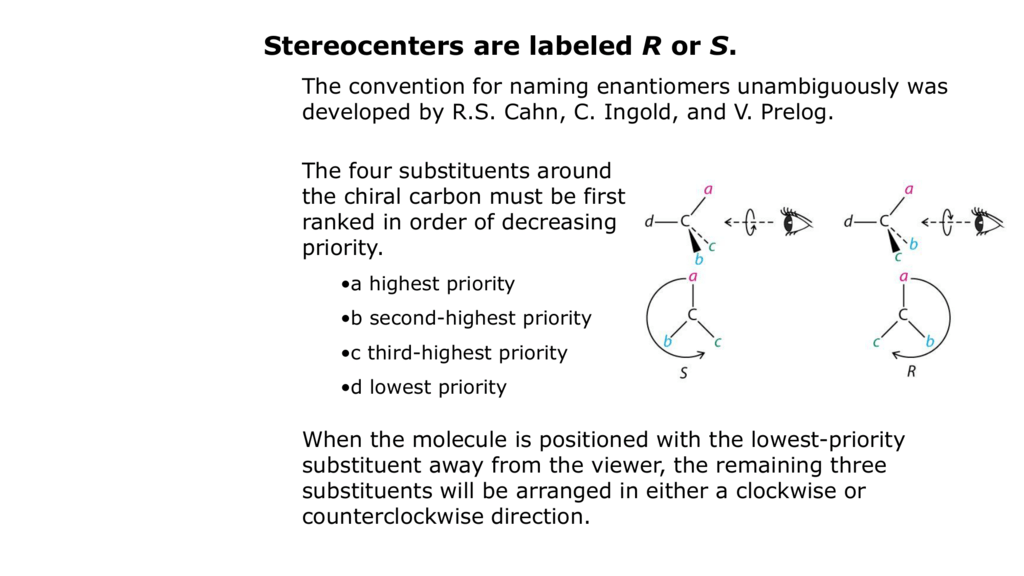



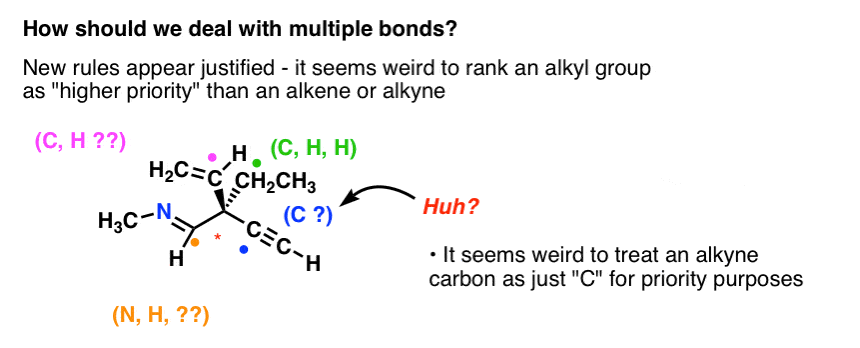
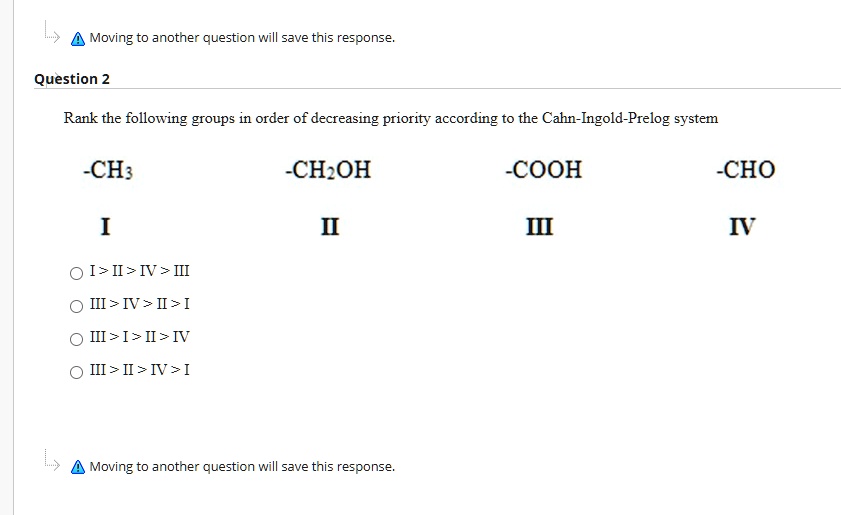

Post a Comment for "Cahn-ingold-prelog System"