Werner Syndrome Cell Signaling
Werner syndrome cell signaling. Aberrant DNA repair has been linked to the development of cancer. Although this has been postulated as causal in the accelerated aging seen in this disease controversy remains as to whether WS is showing the acceleration of a normal cellular. About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works Test new features Press Copyright Contact us Creators.
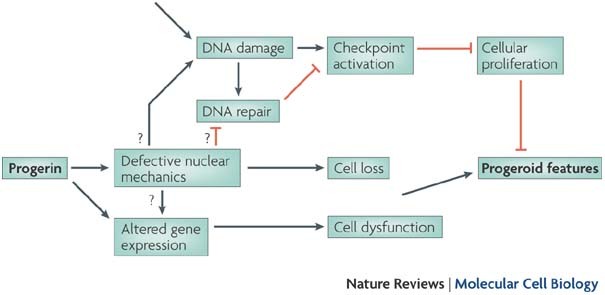
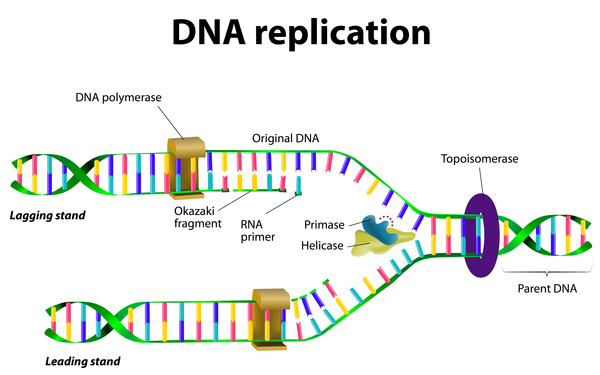
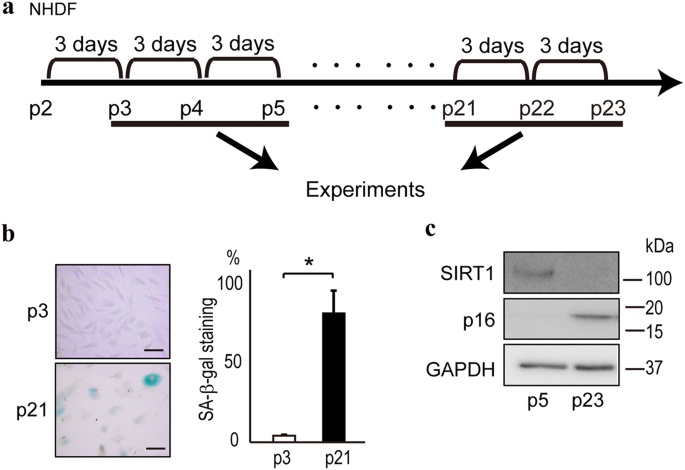
Mutations which cause Werner syndrome all occur at the regions of the gene which encode for protein and not at non-coding regions. Werner syndrome follows an autosomal recessive inheritance pattern which means that a mutation must be present in both copies of the gene for a person to be affected. Werner syndrome WS fibroblasts enter replicative senescence after a reduced in vitro life span.
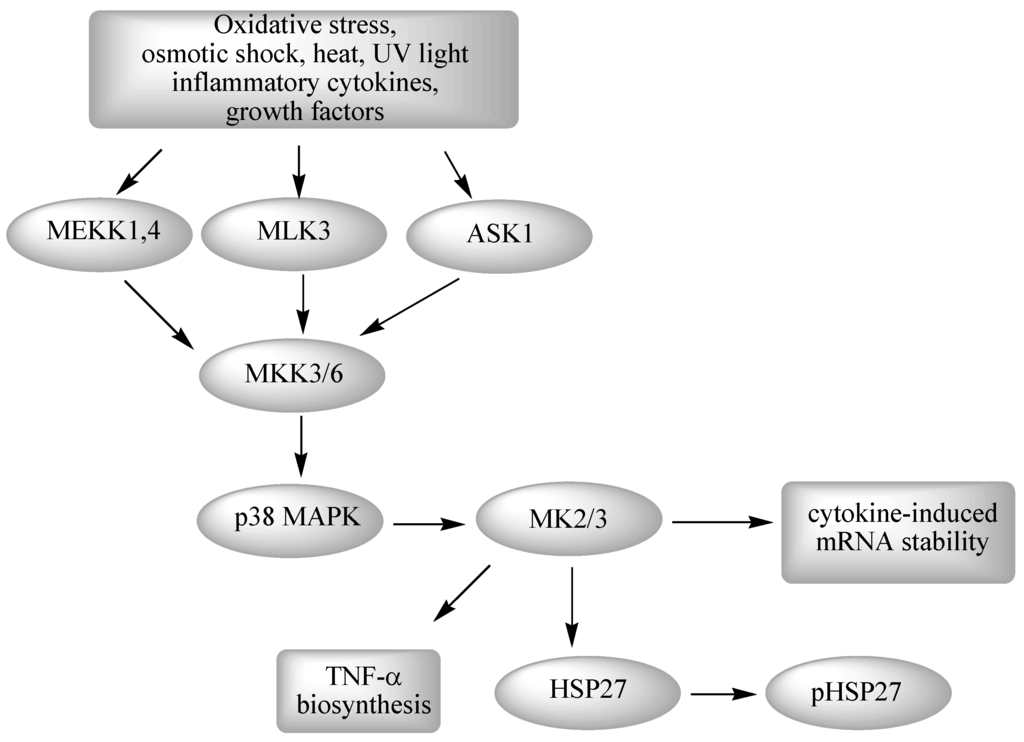
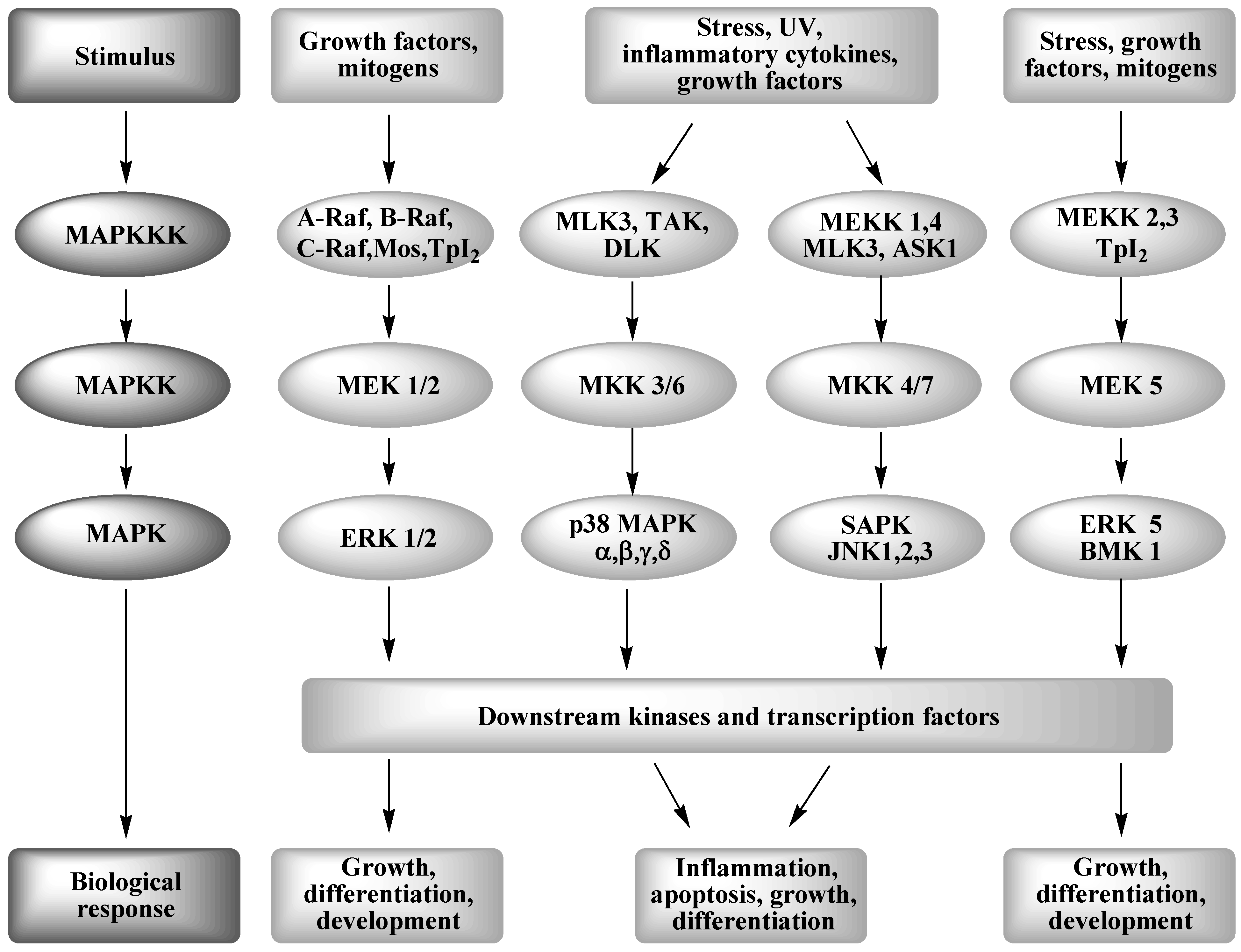
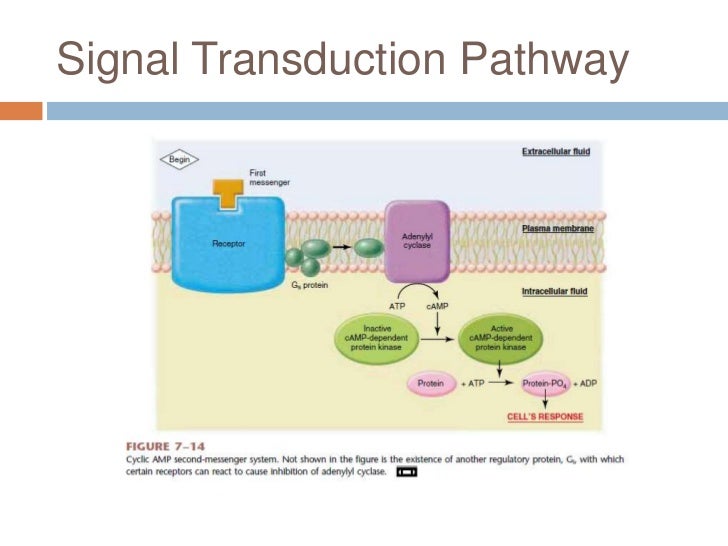
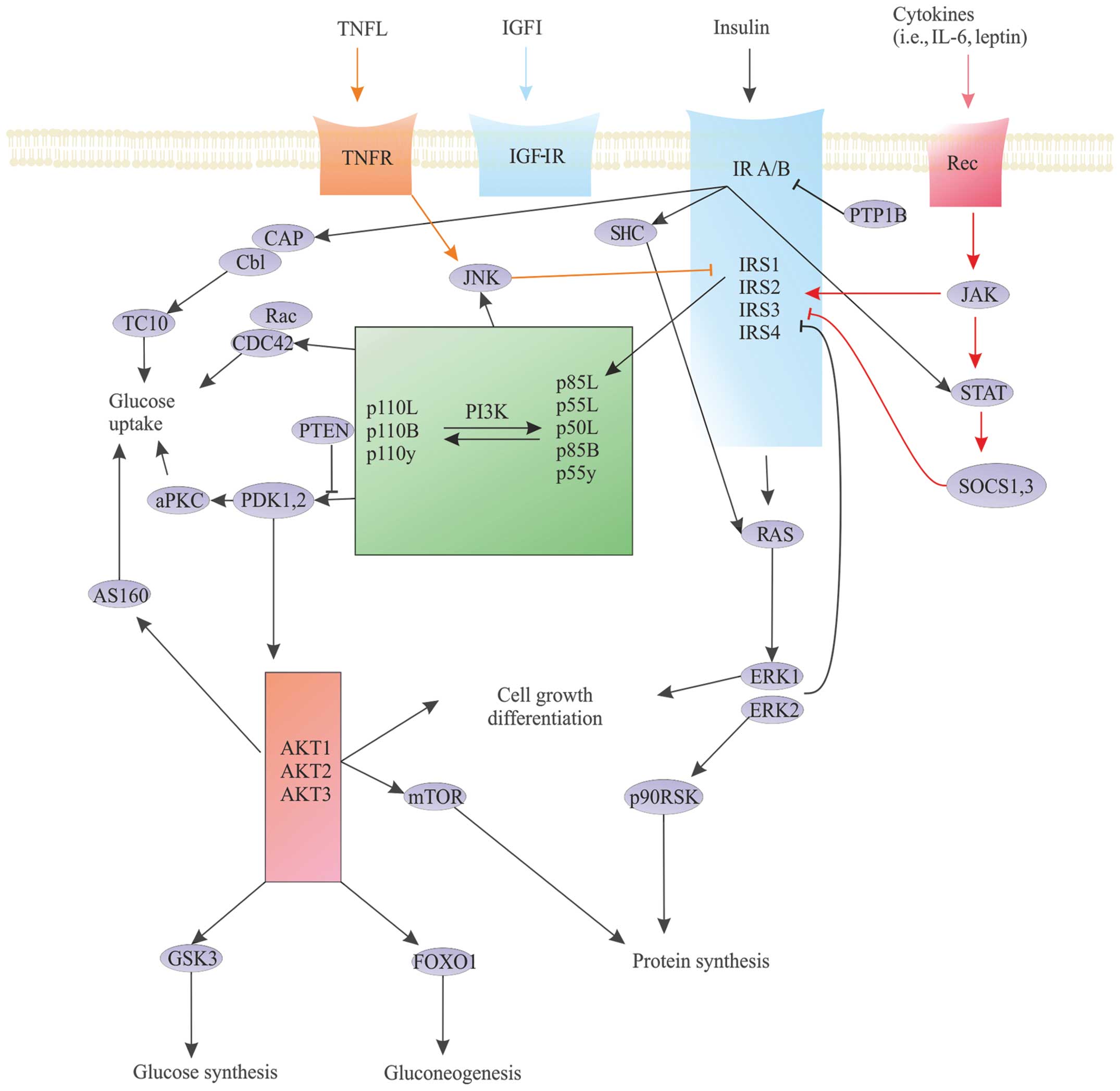
The cause of this lineage-specific aging remains unknown. Reception is the target cells detection of a signaling molecule coming from the outside of the cell. AP Biology - Cell Signaling Diseases Project.
By leveraging recent functional screening data of cancer cell lines we identify Werner syndrome helicase WRN as a novel specific vulnerability of microsatellite instability-high MSI-H cancer cells. WS patients exhibit severe metabolic phenotypes but the underlying mechanisms are not understood and whether the metabolic deficit can be targeted for therapeutic intervention has not been determined. There are 35 different known mutations of WRN which correspond to stop codons insertions or deletions that result in a frameshift mutation.
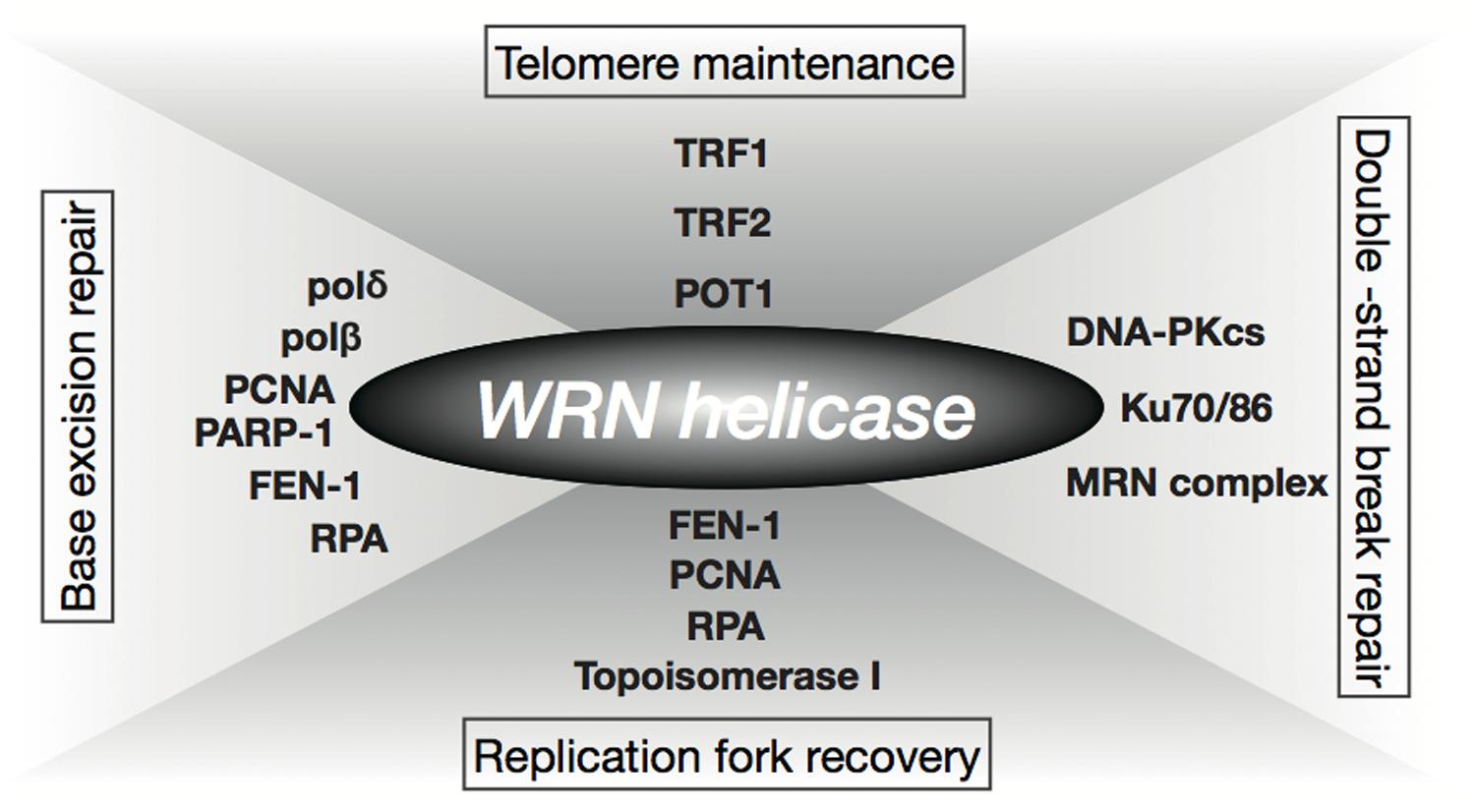
The Werner syndrome protein WRN is a caretaker of the human genome and the Abl kinase is a regulator of the DNA damage response. Metabolic dysfunction is a primary feature of Werner syndrome WS a human premature aging disease caused by mutations in the gene encoding the Werner WRN DNA helicase. Explain the stages of a signal transduction pathway.
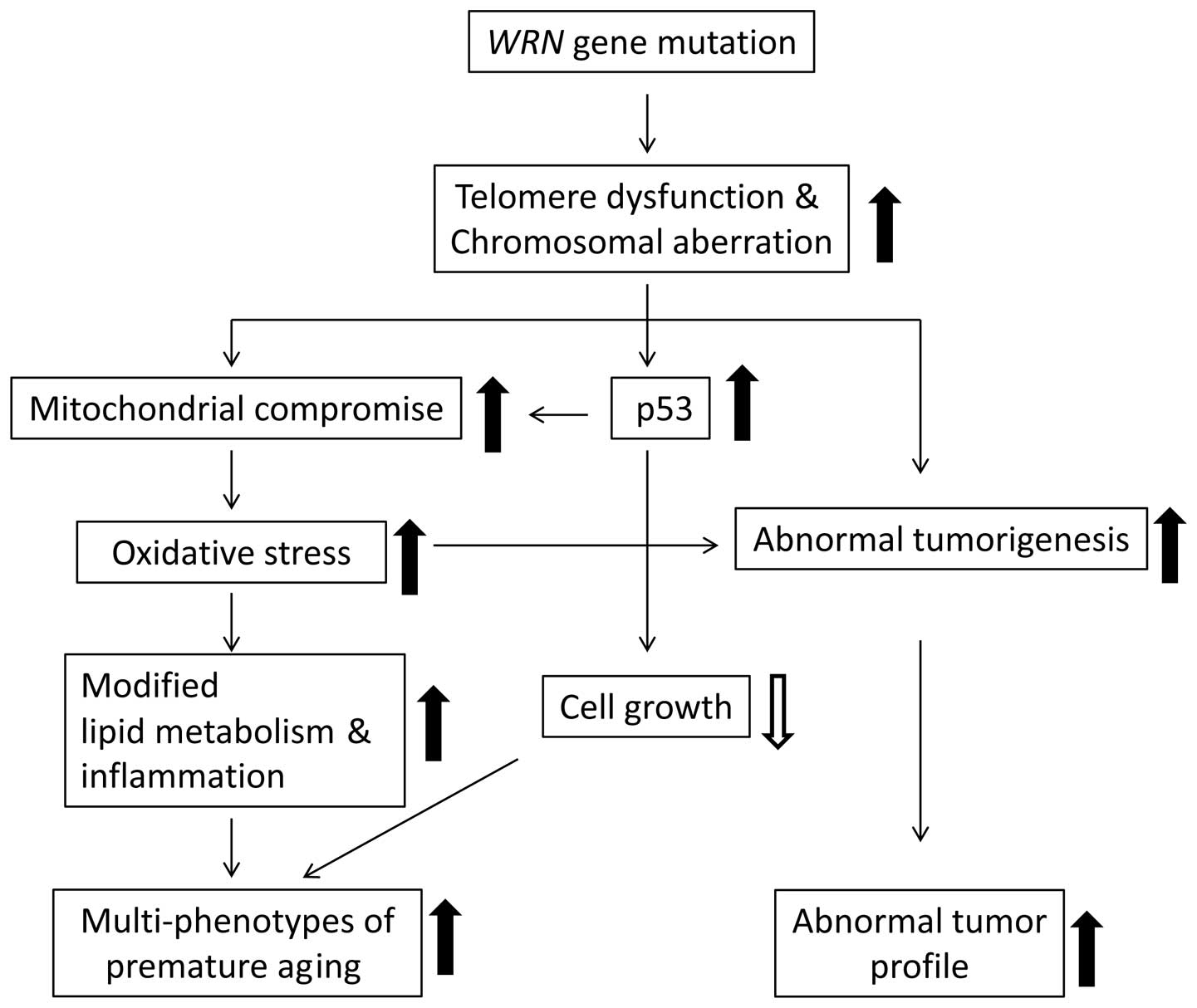
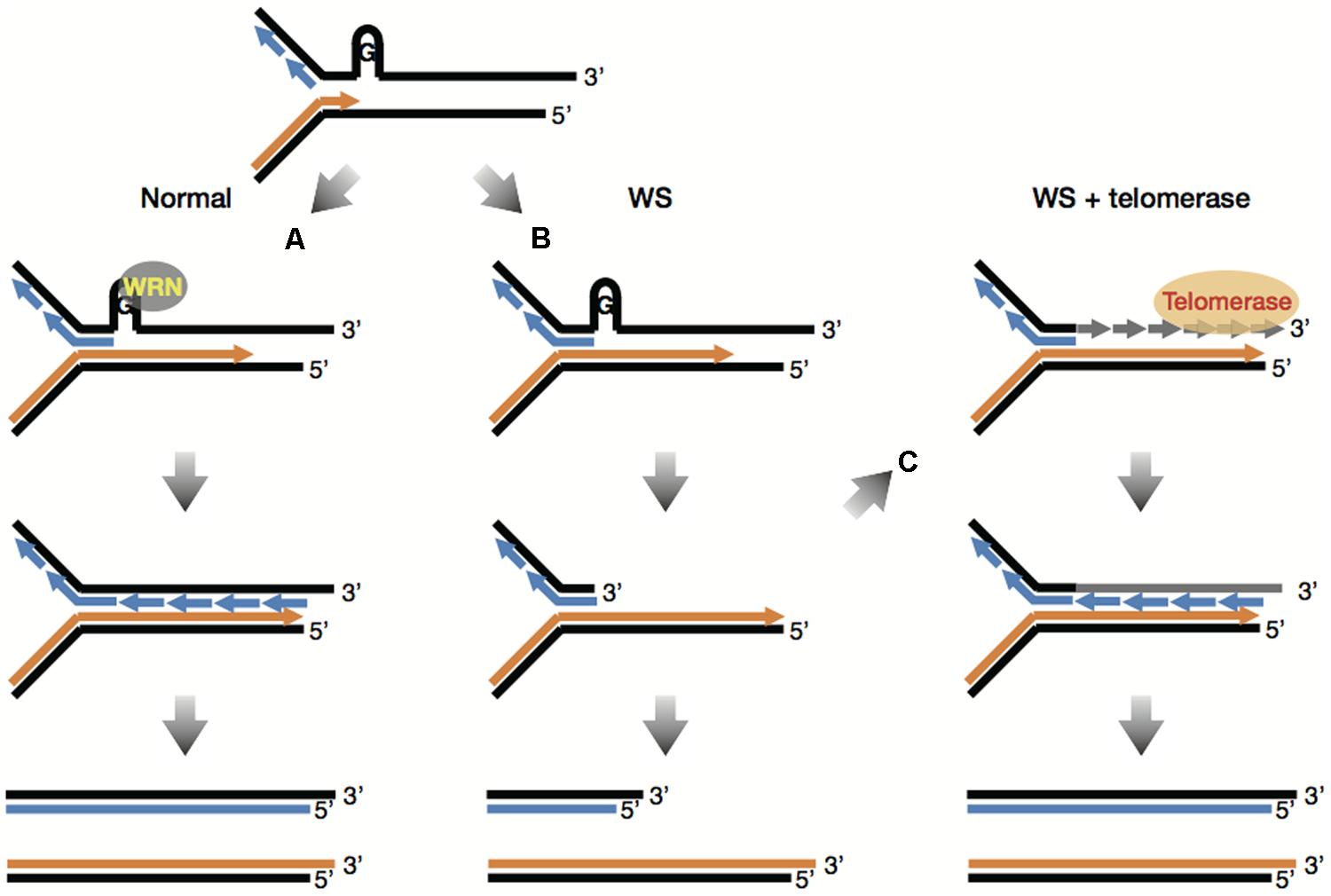
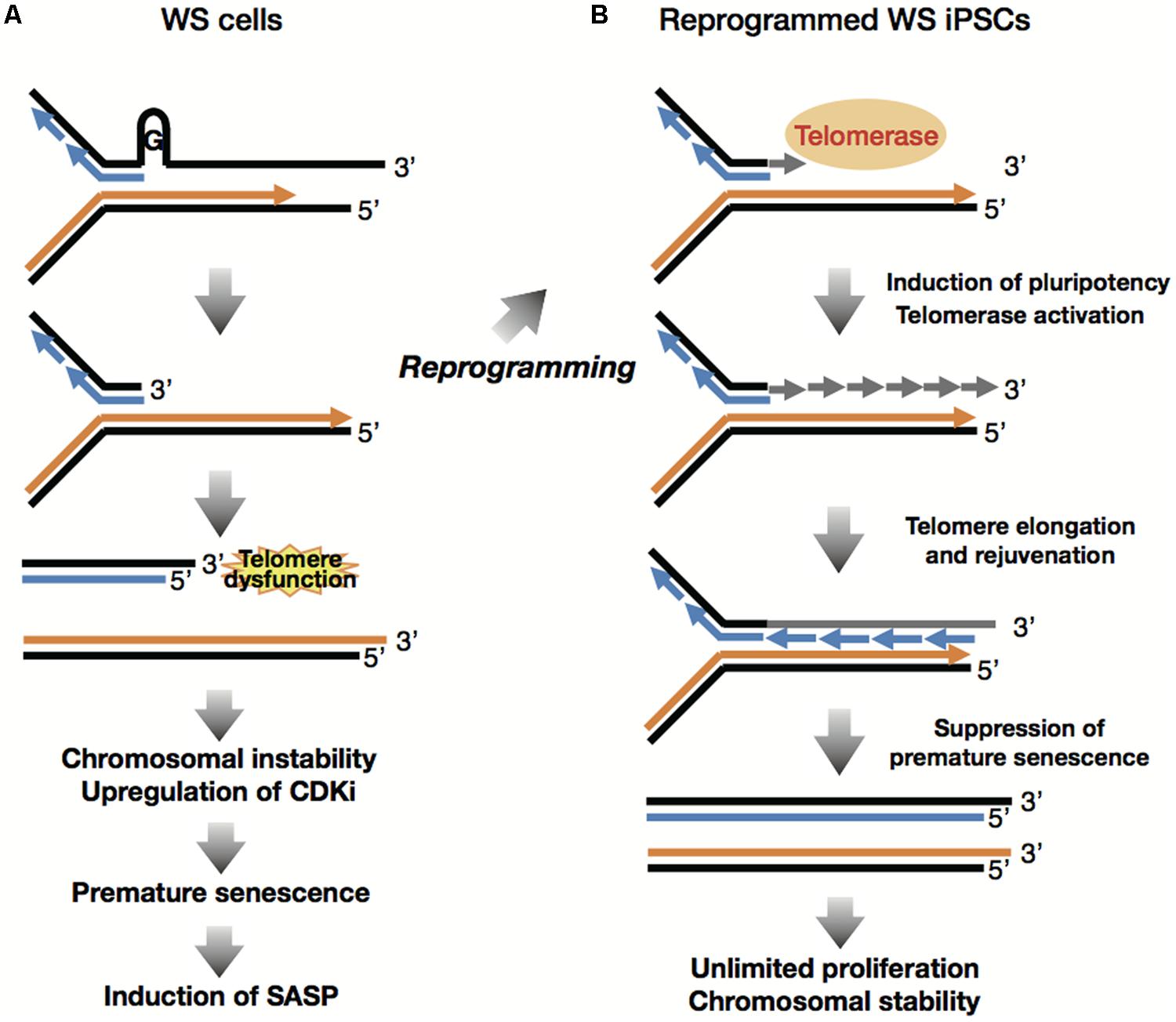
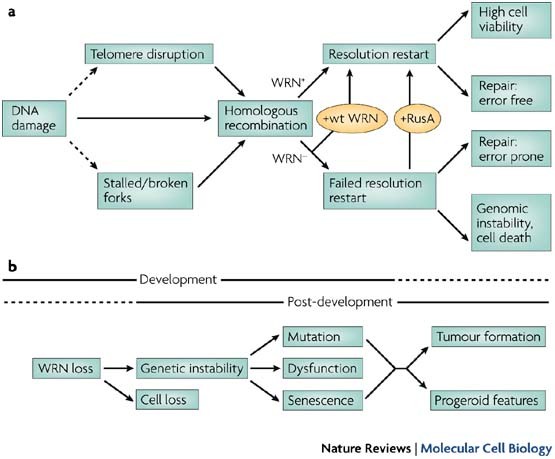
Understand the methods of local and long distance signaling in animals. MSI caused by defective mismatch repair MMR. Werner syndrome WS patients exhibit premature aging predominantly in mesenchyme-derived tissues but not in neural lineages a consequence of telomere dysfunction and accelerated senescence.
PowToon is a free. Werner syndrome is inherited in an autosomal recessive pattern which means both copies of the WRN gene in each cell have mutations.
By leveraging recent functional screening data of cancer cell lines we identify Werner syndrome helicase WRN as a novel specific vulnerability of microsatellite instability-high MSI-H cancer cells.
There are 35 different known mutations of WRN which correspond to stop codons insertions or deletions that result in a frameshift mutation. This means that both parents must pass on a gene mutation for a child to be affected. PowToon is a free. Werner syndrome follows an autosomal recessive inheritance pattern which means that a mutation must be present in both copies of the gene for a person to be affected. Review information about how problems in cell signaling can lead to diseases. Werner syndrome WS fibroblasts enter replicative senescence after a reduced in vitro life span. The cause of this lineage-specific aging remains unknown. Metabolic dysfunction is a primary feature of Werner syndrome WS a human premature aging disease caused by mutations in the gene encoding the Werner WRN DNA helicase. Werner syndrome WS patients exhibit premature aging predominantly in mesenchyme-derived tissues but not in neural lineages a consequence of telomere dysfunction and accelerated senescence.
Werner syndrome WS patients exhibit premature aging predominantly in mesenchyme-derived tissues but not in neural lineages a consequence of telomere dysfunction and accelerated senescence. Here we have identified a direct binding between WRN and c-Abl in vitro via the N-terminal and central regions of WRN and the Src homology domain 3 of c-Abl. Werner syndrome WS patients exhibit premature aging predominantly in mesenchyme-derived tissues but not in neural lineages a consequence of telomere dysfunction and accelerated senescence. This means that both parents must pass on a gene mutation for a child to be affected. Werner syndrome follows an autosomal recessive inheritance pattern which means that a mutation must be present in both copies of the gene for a person to be affected. 66 rows Werner syndrome is inherited in an autosomal recessive pattern. Explain the stages of a signal transduction pathway.
























Post a Comment for "Werner Syndrome Cell Signaling"